![Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY](https://www.touchendocrinology.com/wp-content/uploads/sites/5/2018/02/table1-summary-of-char.png)
Selecting the Appropriate Continuous Glucose Monitoring System – a Practical Approach | [current-page:pager]touchENDOCRINOLOGY

100 years of insulin: celebrating the past, present and future of diabetes therapy | Nature Medicine

Clinical Evidence Supporting FDA Clearance of First-of-a-Kind Therapeutic Devices via the De Novo Pathway Between 2011 and 2019 | medRxiv
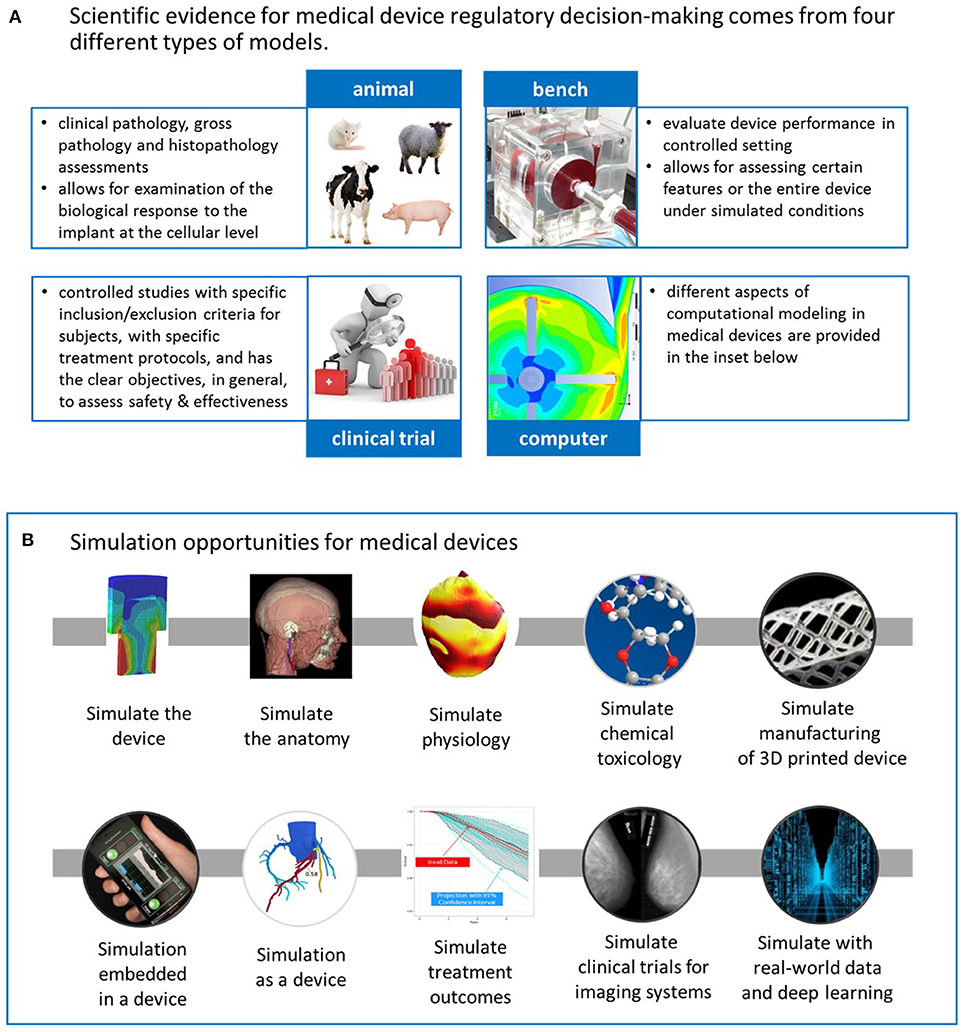
Frontiers | Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA's Office of Science and Engineering Laboratories

The Food and Drug Administration's (FDA's) 510(k) Process: A Systematic Review of 1000 Cases - The American Journal of Medicine
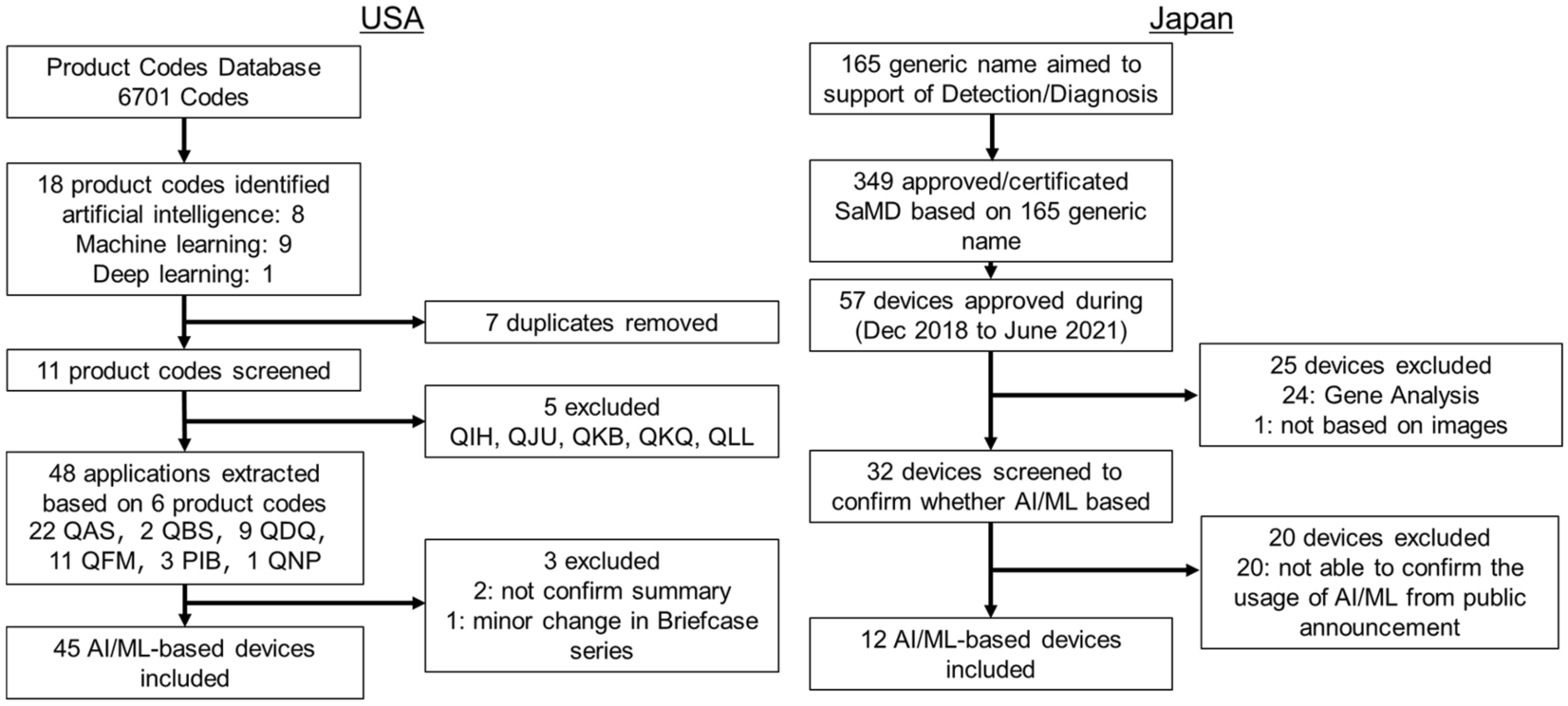
Systematic analysis of the test design and performance of AI/ML-based medical devices approved for triage/detection/diagnosis in the USA and Japan | Scientific Reports
510(k) SUMMARY OF SAFETY AND EFFECTIVENESS Deltec CozmoTM Insulin Infusion Pump (Model 1700) and Accessories I. GENERAL INFORMAT

Study Design and Data Analysis of Artificial Pancreas Device Systems with Closed-Loop Glucose-Sensing Insulin Delivery